Water chemistry is a branch of chemistry that deals with the chemical properties and behavior of water. It encompasses the study of the composition, structure, and reactions of water molecules and the substances dissolved in water. One of the fundamental aspects of water chemistry is understanding its unique properties, such as its ability to dissolve a wide range of substances due to its polar nature and its role as a universal solvent. Additionally, water chemistry examines the interactions between water and other substances, including acids, bases, salts, and organic compounds.
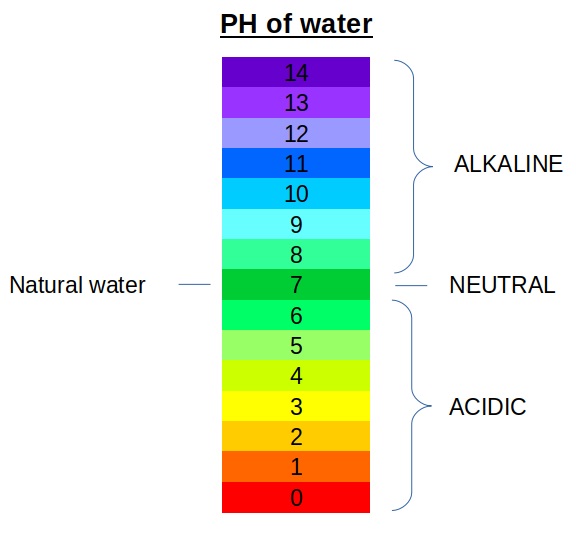
pH is a crucial concept in water chemistry, representing the acidity or alkalinity of a solution. The pH scale ranges from 0 to 14, with 7 being neutral, below 7 acidic, and above 7 alkaline. Natural water bodies, such as rivers, lakes, and oceans, have varying pH levels influenced by factors like geological formations, atmospheric deposition, and human activities. Changes in pH can have significant impacts on aquatic ecosystems, affecting the health of aquatic organisms and the overall water quality.
Another important aspect of water chemistry is the presence of dissolved ions and molecules, which contribute to the water’s properties and behavior. Common ions found in water include calcium, magnesium, sodium, chloride, and sulfate. The concentrations of these ions can affect various processes, such as the hardness of water, precipitation reactions, and the formation of scale in industrial processes. Understanding the composition and behavior of dissolved substances in water is essential for addressing issues related to water quality, treatment, and environmental management. Water chemistry plays a critical role in ensuring the sustainable use and management of water resources for various purposes, including drinking water supply, agriculture, industry, and recreation.
What about water chemistry interesting facts? Let’s take a look at these 10 interesting facts about water chemistry.
- Polarity and Hydrogen Bonding : Water molecules are polar, meaning they have a positive and negative end. This polarity allows water molecules to form hydrogen bonds with each other, giving water its unique properties such as surface tension, cohesion, and adhesion.
- pH Regulation : The pH of water determines its acidity or alkalinity. Natural water bodies typically have a pH ranging from about 6.5 to 8.5. Aquatic organisms are highly sensitive to changes in pH, and slight fluctuations can have significant impacts on their health and survival.
- Hard Water vs. Soft Water : Water hardness refers to the concentration of dissolved minerals, primarily calcium and magnesium ions. Hard water can cause scale buildup in pipes and appliances, while soft water is less likely to leave deposits but may require additional treatment for drinking purposes.
- Water’s Solvent Properties : Water is often called the “universal solvent” because of its ability to dissolve a wide range of substances. This property is essential for processes like hydration, nutrient transport in organisms, and the weathering of rocks.
- Water’s Density Anomaly : Unlike most substances, water reaches its maximum density at around 4°C (39.2°F) and becomes less dense as it freezes into ice. This anomaly is crucial for aquatic ecosystems as it allows ice to float, insulating the water below and preserving life during cold seasons.
- Hydrolysis Reactions : Water participates in hydrolysis reactions, where it breaks down molecules by adding water molecules to their chemical structure. Hydrolysis is involved in various biological processes, including digestion, metabolism, and the breakdown of complex molecules into simpler ones.
- Water and Acid-Base Chemistry : Water can act as both an acid and a base, undergoing self-ionization to produce hydronium ions (H3O+) and hydroxide ions (OH-). This equilibrium is vital for maintaining the pH balance in aqueous solutions.
- Water Treatment Processes : Water chemistry is central to water treatment processes, including coagulation, flocculation, sedimentation, filtration, and disinfection. These processes aim to remove contaminants and pathogens from water to make it safe for drinking and other purposes.
- Aquatic Redox Reactions : Redox (reduction-oxidation) reactions play a crucial role in aquatic environments, influencing the cycling of elements like oxygen, carbon, nitrogen, and sulfur. These reactions involve the transfer of electrons between substances and are vital for energy production and nutrient cycling in ecosystems.
- Environmental Impact : Human activities, such as industrial pollution, agriculture, and urbanization, can significantly alter water chemistry. Pollution from chemicals, heavy metals, and nutrients can degrade water quality, leading to ecological imbalances, habitat destruction, and threats to human health. Understanding water chemistry is essential for mitigating these impacts and ensuring the sustainable management of water resources.
Water chemistry serves as a cornerstone of understanding the intricate behaviors and properties of one of Earth’s most vital resources. From its unique solvent abilities to its role in maintaining pH balance and facilitating essential biological processes, water chemistry plays a fundamental role in various fields, including environmental science, biology, and engineering. As we continue to face challenges such as pollution, climate change, and growing water demands, a comprehensive understanding of water chemistry becomes increasingly critical. By leveraging this knowledge, we can strive towards sustainable water management practices, safeguarding both the health of ecosystems and the well-being of communities worldwide.